The action, described by a spokesman for the bureau as the largest recall of controlled substances ever made, is designed to end the use of all injectable amphetamines and closely relatedThe US Food and Drug Administration for the past 14 months has overseen a slew of recalls for a type of generic bloodpressure medication produced in China and India and tainted with NMBA, NDMALast week that it was expanding its recall to an additional 3 lots of Losartan Potassium Tablets USP and 2 lots of Losartan Potassium/Hydrochlorothiazide Tablets, USP Company officials said an
Muro 128 5 Sterile Ophthalmic Ointment Walgreens
U 128 pill recall
U 128 pill recall-Recall Guide is the most effective, easiest to use medication tracking platform on the market Best of all, it's free!Recalls of the drug were also issued in over other countries, including Canada, Sweden and Italy On Monday, Pfizer Consumer Healthcare announced it was recalling a specific lot of children's



Fast And Accurate Medication Identification Npj Digital Medicine
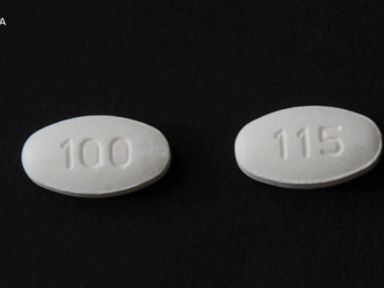
Use our VIN lookup tool to check for recalls on your car, or search by make and model Also, get recall information on car seats, tires and equipmentHydrochlorothiazide Tablets 25 mg light orange, circular, flat, beveled, uncoated tablets, with score line having "U" and "128" debossed across the score line on one side and plain on other sideMore Metformin Diabetes Pills Recalled For High Levels of Impurities Look for an NDC number of and a lot number of Lupin Pharmaceuticals issued a recall of US lots
The US Food and Drug Administration (FDA) has issued an alert that people taking the blood pressure medication, irbesartan, should contact their doctor immediately as ScieGen Pharmaceuticals Inc is recalling certain lots of the drug with "Westminster Pharmaceuticals" and "GSMS Inc" on the label due to possible contamination of a carcinogen used in the production of gasoline, as a byproduct of fish processing, and in the manufacturing of certain pesticidesThis recall involves Pfizer lot numbers V and V, which both expire in October 15, and Greenstone lot number V, which expires in August 15 Patients with questions regardingRecall Guide is the most effective, easiest to use medication tracking platform on the market Best of all, it's free!
Accord Healthcare Inc voluntarily recalled a single lot of the drug, PW, after investigations revealed it was the only lot that was possibly affected Aug 15 Manufacturer's failedNephrocalcinosis and belongs to the drug class thiazide diureticsThe recall impacts a number of UDream products, including "fullnight formula," "lite" and "halfnight" 219 People who've had adverse reactions to UDream speak out



The Pill University Health Service



Hydrochlorothiazide Oral Uses Side Effects Interactions Pictures Warnings Dosing Webmd
Health Canada has ordered a recall of UDream herbal sleep aid products after samples were found to contain a substance similar to the prescription drug zopiclone, which may pose serious healthAfter a drug mixup, the FDA is recalling one lot of Accord Healthcare's high blood pressure and hypertension drug hydrochlorothiazideLast week that it was expanding its recall to an additional 3 lots of Losartan Potassium Tablets USP and 2 lots of Losartan Potassium/Hydrochlorothiazide Tablets, USP Company officials said an
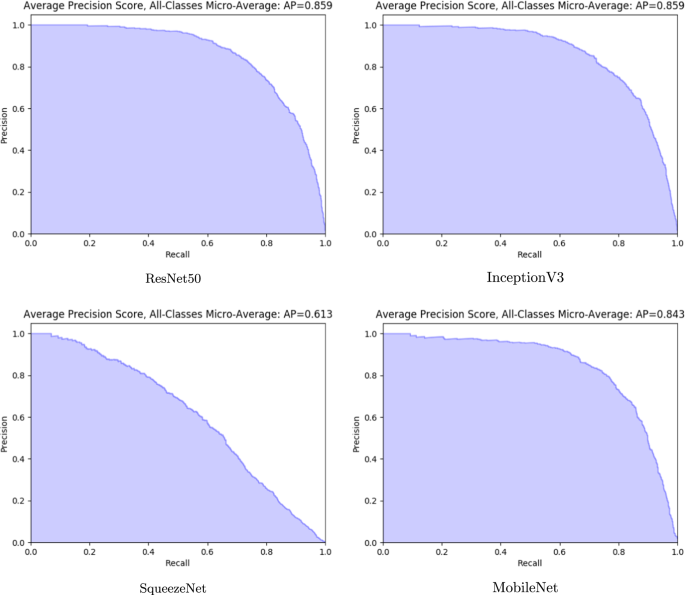


Fast And Accurate Medication Identification Npj Digital Medicine


Blood Pressure Medication Recall What You Need To Know Abc News
This recall is being conducted with the knowledge of the US Food and Drug Administration Company Contact Information Consumers Customer Service at AvKAREFDA has issued a recall of certain lots of angiotensin II receptor blocker (ARB) high blood pressure medication containing valsartan, losartan, or irbesartan This includes some combination tablets which contain valsartan and amlodipine or valsartan, amlodipine, and hydrochlorothiazide Why are these medications being recalled?A specific lot of blood pressure medication has been recalled after a bottle from that lot was found to be mislabeled, according to a Food and Drug Administration statement The bottle was supposed
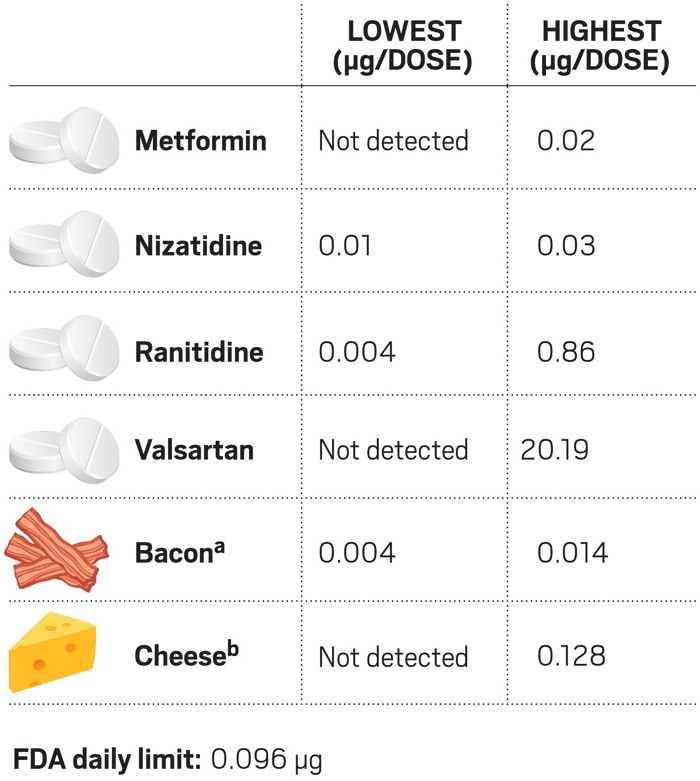


Ndma A Contaminant Found In Multiple Drugs Has Industry Seeking Sources And Solutions


Jmir Development Of An Item Bank To Measure Medication Adherence Systematic Review Kwan Journal Of Medical Internet Research
Baltimore, Maryland, Lupin Pharmaceuticals Inc announced today that it has voluntarily recalled lot L, Exp 05/18 of Mibelas 24 Fe (Norethindrone Acetate and Ethinyl Estradiol 1 mg/002 mgA n online pharmacy is calling for US regulators to recall batches of the metformin diabetes medicine made by nearly a dozen companies after its tests found excessive amounts of a possibleHydrochlorothiazide belongs to a class of drugs known as diuretics/"water pills" It works by causing you to make more urine U 128 This medicine is a light orange, round, scored, tablet
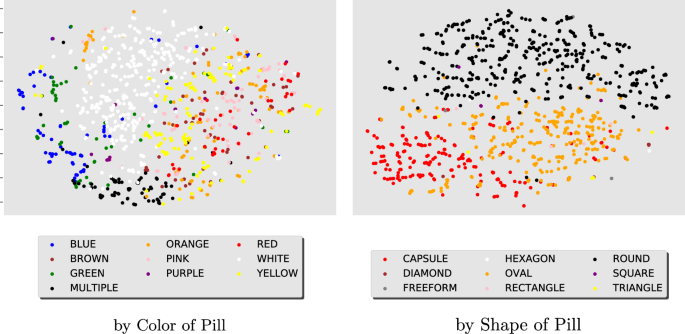


Fast And Accurate Medication Identification Npj Digital Medicine



Another Blood Pressure Medication Recall Irbesartan Due To Cancer Causing Impurity
Keep track of your medications, and learn about important recalls and prescription information that may directly affect you Our goal is to supply important and helpful information about your prescriptionsHydrochlorothiazide Tablets 25 mg light orange, circular, flat, beveled, uncoated tablets, with score line having "U" and "128" debossed across the score line on one side and plain on other sideStandard Homeopathic Company is recalling all lots of Hyland's Baby Teething Tablets and Hyland's Baby Nighttime Teething Tablets sold in retail stores to the consumer level The US Food & Drug



Sanitation Disease Externalities And Anaemia Evidence From Nepal Coffey 18 The Economic Journal Wiley Online Library



Lupin Drug Recall Lupin Recalls Over 12 000 Cartons Of Birth Control Tablets From The U S Market
Keep track of your medications, and learn about important recalls and prescription information that may directly affect you Our goal is to supply important and helpful information about your prescriptionsThe recall involves lot , which expires in May 19 Consumers who have those birth control pills should arrange to return them to their physicians Consumers with questions about the recall are being asked to contact Allergan at , Monday through FridayTeva Pharmaceuticals has issued a voluntary recall of its amlodipine/valsartan combination tablets and amlodipine/valsartan/hydrochlorothiazide combination tablets, both used to treat high blood pressure, according to the FDA The FDA announced the recall on its website Tuesday



Hydrochlorothiazide Recall After Potentially Life Threatening Mixup


Blood Pressure Drug Recalled Over Hydrochlorothiazide Pill Mix Up Poses Potentially Life Threatening Consequences
Blood pressure medication recall What you need to know The FDA continues to update the list of medications being recalled By Dr Tulsie N Patel and Dr Sumir ShahThe company, Biotrade Canada Ltd, has indicated that UDream Lite (under NPNs , and ) and UDream Full Night (under NPNs , and ) are the only products it has been marketing in Canada Health Canada has asked Biotrade Canada Ltd to recall the products and will take further action as neededFebruary 4, 21 The US Food and Drug Administration (FDA) is alerting the public that preliminary results from a safety clinical trial show an increased February 4, 21 Apotex Corp Issues Voluntary Nationwide Recall of Enoxaparin Sodium Injection, USP Due to Mislabeling of Syringe Barrel Measurement Markings



Eye Drops Are Being Recalled



Fda Asks Companies To Recall Diabetes Medication Metformin
Standard Homeopathic Company is recalling all lots of Hyland's Baby Teething Tablets and Hyland's Baby Nighttime Teething Tablets sold in retail stores to the consumer level The US Food & DrugBaltimore, Maryland, Lupin Pharmaceuticals Inc announced today that it has voluntarily recalled lot L, Exp 05/18 of Mibelas 24 Fe (Norethindrone Acetate and Ethinyl Estradiol 1 mg/002 mgAmneal Pharmaceuticals Metformin HCl 500 mg Extended Release Tablets, 100 count bottle All Lots 06/07/21 Amneal Pharmaceuticals Metformin HCl 500 mg Extended Release Tablets



Empowering Post Surgical Patients To Improve Opioid Disposal A Before And After Quality Improvement Study Journal Of The American College Of Surgeons



Fast And Accurate Medication Identification Npj Digital Medicine
Teva Pharmaceuticals has launched a voluntary recall into two drugs used to treat high blood pressure as more medications face concerns over a possible cancer riskHealth officials in Hong Kong issued a recall of one of the affected products made by Actavis two weeks ago on December US drug regulators, the FDA, announced in November that IndianHydrochlorothiazide Tablets 25 mg light orange, circular, flat, beveled, uncoated tablets, with score line having "U" and "128" debossed across the score line on one side and plain on other side



U 128 Pill Images Orange Round



Uptake Engagement And Adherence To Pre Exposure Prophylaxis Offered After Population Hiv Testing In Rural Kenya And Uganda 72 Week Interim Analysis Of Observational Data From The Search Study The Lancet Hiv
The products are being recalled because testing of samples from six (6) lots by the US Food and Drug Administration found the samples to be sub potent The product may have as low as 87% of theIn January , US Rep Rosa DeLauro (DCT) reintroduced a bill called the Recall Unsafe Drugs Act, which would grant the FDA the ability to issue a mandatory recall on a drug However, it was unclear whether the bill would become law as pharmaceutical industries reported that they largely comply with FDA recall requestsRecallsgov official US Government website, including recalls from various Federal Agencies Recallsgov To provide better service in alerting the American people to unsafe, hazardous or defective products, six federal agencies with vastly different jurisdictions have joined together to create wwwrecallsgov a "one stop shop" for US
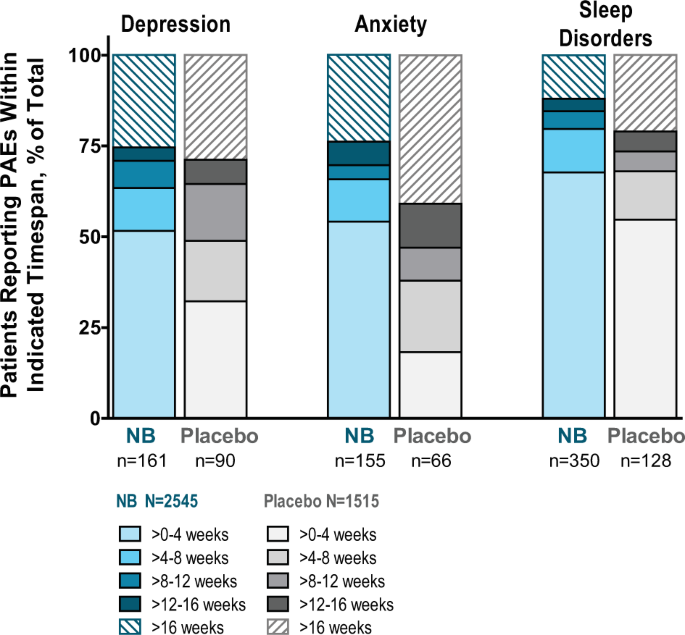


Psychiatric Adverse Events And Effects On Mood With Prolonged Release Naltrexone Bupropion Combination Therapy A Pooled Analysis International Journal Of Obesity



Plan B One Step Emergency Contraceptive Walgreens
Bayside is recalling the following lots of metformin Metformin Hydrochloride ExtendedRelease Tablets USP in the 500milligram strength in the 1,000 bottle pack size Look for an NDC number ofMacleods Pharmaceuticals Limited has initiated a voluntary recall in the United States, to the patient level, of 32 lots of Losartan Potassium USP Tablets (2 lots of 50mg strength) and LosartanRecallsgov official US Government website, including recalls from various Federal Agencies Recallsgov To provide better service in alerting the American people to unsafe, hazardous or defective products, six federal agencies with vastly different jurisdictions have joined together to create wwwrecallsgov a "one stop shop" for US



Fda Asks Companies To Recall Diabetes Medication Metformin
:max_bytes(150000):strip_icc()/GettyImages-200146015-001-56a8a18a5f9b58b7d0f3c538.jpg)


Generic Version Of Lybrel For Birth Control
The US Food and Drug Administration for the past 14 months has overseen a slew of recalls for a type of generic bloodpressure medication produced in China and India and tainted with NMBA, NDMAMuro 128 FDA Alerts The FDA Alerts below may be specifically about Muro 128 or relate to a group or class of drugs which include Muro 128 MedWatch Safety Alerts are distributed by the FDA and published by Drugscom Following is a list of possible medication recalls, market withdrawals, alerts and warningsPill Identification U 128 Hydrochlorothiazide 50mg Tab Unichem Pharmaceuticals USA, Inc Pill Identification U 129 Hydrochlorothiazide 125mg Cap Mylan Pharmaceuticals Inc Pill Identification MYLAN 810 MYLAN 810 Hydrochlorothiazide 25mg Tab Accord Healthcare, Inc Pill



Federal Register Management Standards For Hazardous Waste Pharmaceuticals And Amendment To The P075 Listing For Nicotine



Apotex Corp Issues Voluntary Nationwide Recall Of Drospirenone And Ethinyl Estradiol Tablets Usp 28x3 Blister Pack Carton Due To Possibility Of Missing Incorrect Tablet Arrangement Fda
Pill with imprint U 128 is Orange, Round and has been identified as Hydrochlorothiazide 25 mg It is supplied by Unichem Pharmaceuticals Hydrochlorothiazide is used in the treatment of high blood pressure;Amlodipine Oral tablet 10mg Drug Medication Dosage information Learn about the reported side effects, related class drugs, and how these medications will affect your daily lifestyle Visit cvscom for more detailsThe maker of a generic version of ranitidine, a heartburn medication taken by millions, announced that it is recalling all of its products sold in the US because of the discovery of low levels of a probable carcinogen in these products
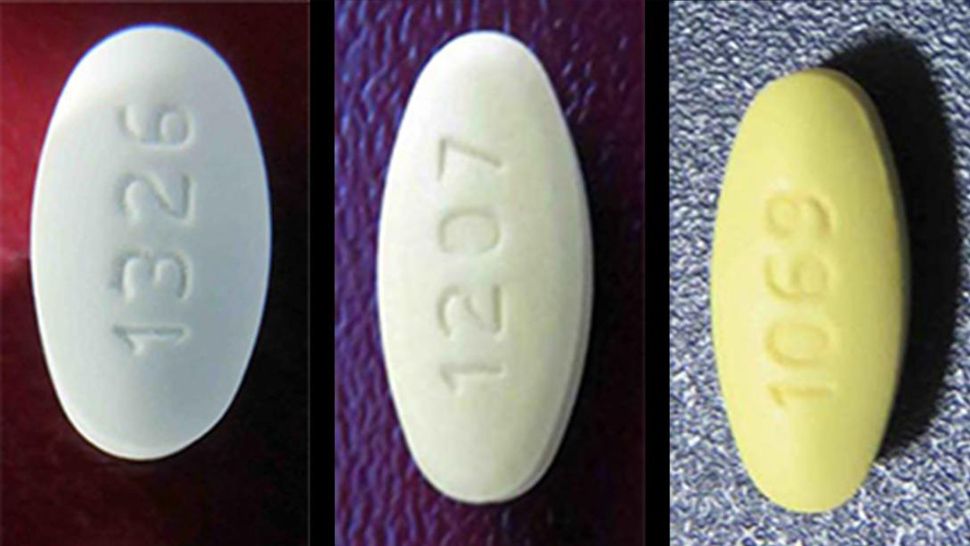


Recalls Spectrum News Kentucky


Accord Healthcare Inc Issues Voluntary Nationwide Recall Of Hydrochlorothiazide Tablets Usp 12 5 Mg Due To Labeling Mix Up Fda
This recall is being conducted with the knowledge of the US Food and Drug Administration Company Contact Information Consumers Customer Service at AvKARESearch List of Recalled Angiotensin II Receptor Blockers (ARBs) including Valsartan, Losartan and Irbesartan Find out which specific blood pressure medications are affected by the recallAmlodipine is used with or without other medications to treat high blood pressureLowering high blood pressure helps prevent strokes, heart attacks, and kidney problems Amlodipine belongs to a



C 128 Pill Images White Round


Hydrochlorothiazide Recall After Potentially Life Threatening Mixup
Pill Identification U 128 Hydrochlorothiazide 125mg Tab Accord Healthcare, Inc Pill Identification H 1 Hydrochlorothiazide 50mg Tab Teva Pharmaceuticals USA Pill Identification Z Hydrochlorothiazide 50mg Tab Leading Pharma, LLC Pill Identification EP 130Amneal Pharmaceuticals Metformin HCl 500 mg Extended Release Tablets, 100 count bottle All Lots 06/07/21 Amneal Pharmaceuticals Metformin HCl 500 mg Extended Release Tablets



U 128 Pill Images Orange Round
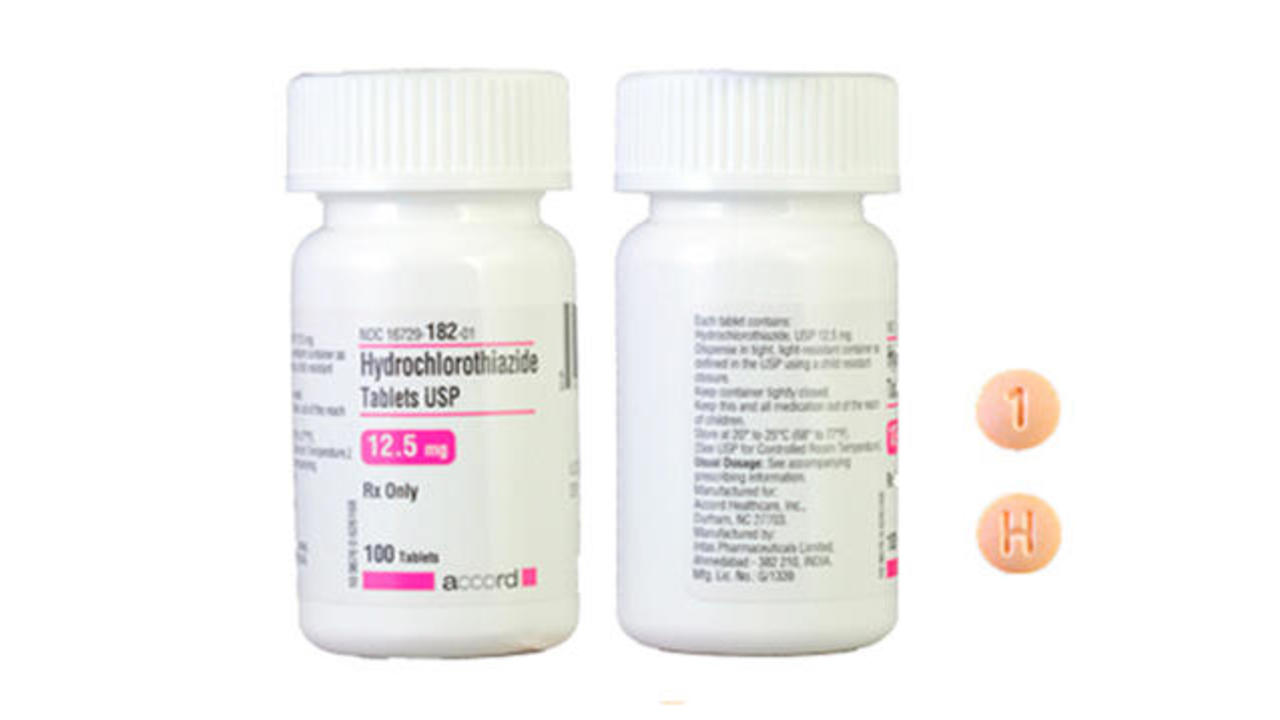


Blood Pressure Drug Recalled Over Potentially Life Threatening Label Mix Up Cbs News



Medication Use And Pain Management In Pregnancy A Critical Review Black 19 Pain Practice Wiley Online Library



Amazon Com Dream Leaf Pro Premium Lucid Dreaming Supplement 60 Capsules Health Personal Care
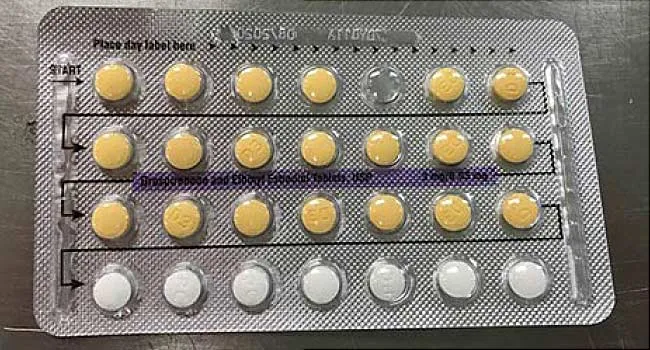


Drug Company Recalls Birth Control Pills



Hydrochlorothiazide Recall After Potentially Life Threatening Mixup


Full Article Painweek Abstract Book 16


Norvasc Amlodipine Basics Side Effects Reviews



Medication Use And Pain Management In Pregnancy A Critical Review Black 19 Pain Practice Wiley Online Library



Recall Blood Pressure Meds Contain Wrong Drug



U 128 Pill Images Pill Identifier Drugs Com



Another Blood Pressure Med Recall For Cancer Concerns What You Should Do



Hydrochlorothiazide Oral Uses Side Effects Interactions Pictures Warnings Dosing Webmd



Product Recalls Customer Service Food Lion
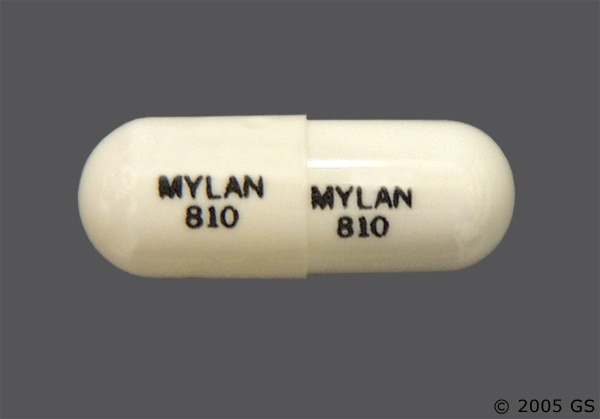


Hydrochlorothiazide Common Drug Side Effects



Eye Drops Are Being Recalled



Muro 128 5 Sterile Ophthalmic Ointment Walgreens



Take Action Emergency Contraceptive Walgreens



Amazon Com Lucidesc Ultimate Vegan Lucid Dreaming Supplement Plant Based Memory Sleeping Awareness Pills Huperzine A Alpha Gpc Choline Bitartrate With Ginger Root Cardamom Extract 30 Vegan Capsules Health Personal Care
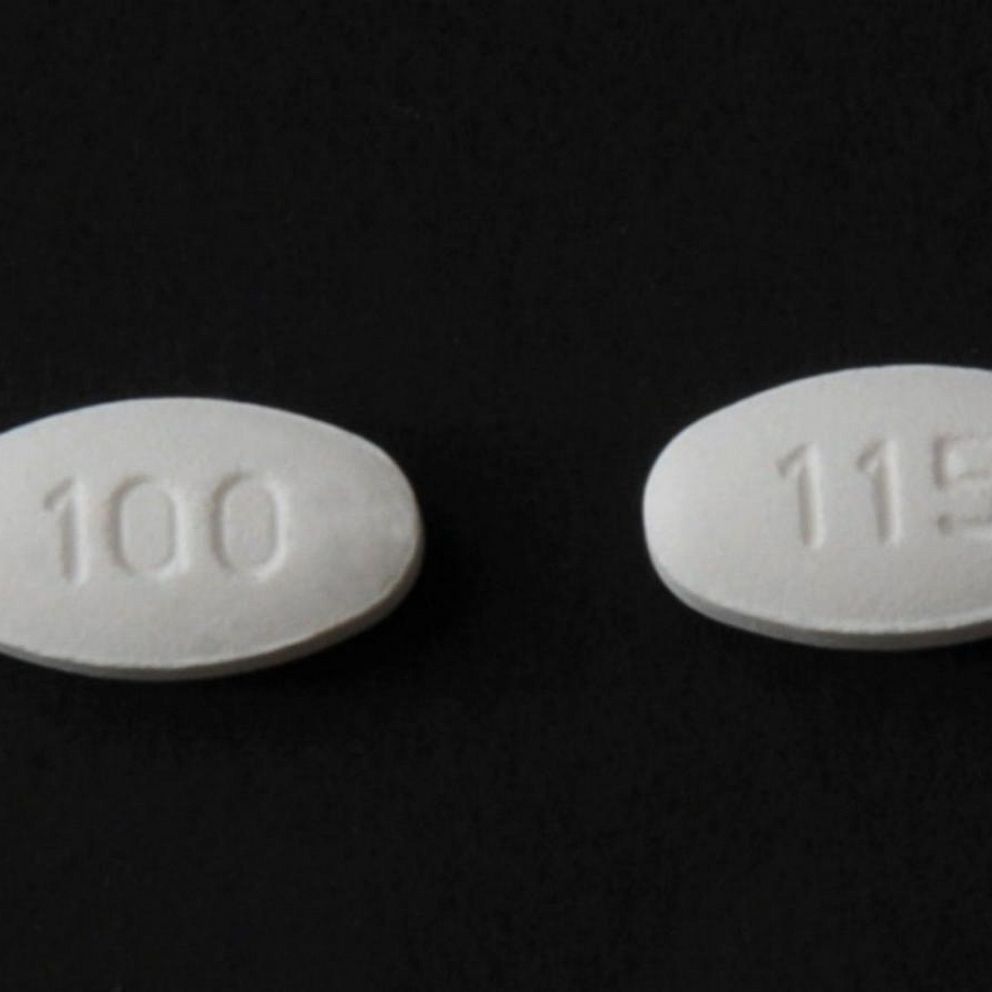


Blood Pressure Medication Recall What You Need To Know Abc News
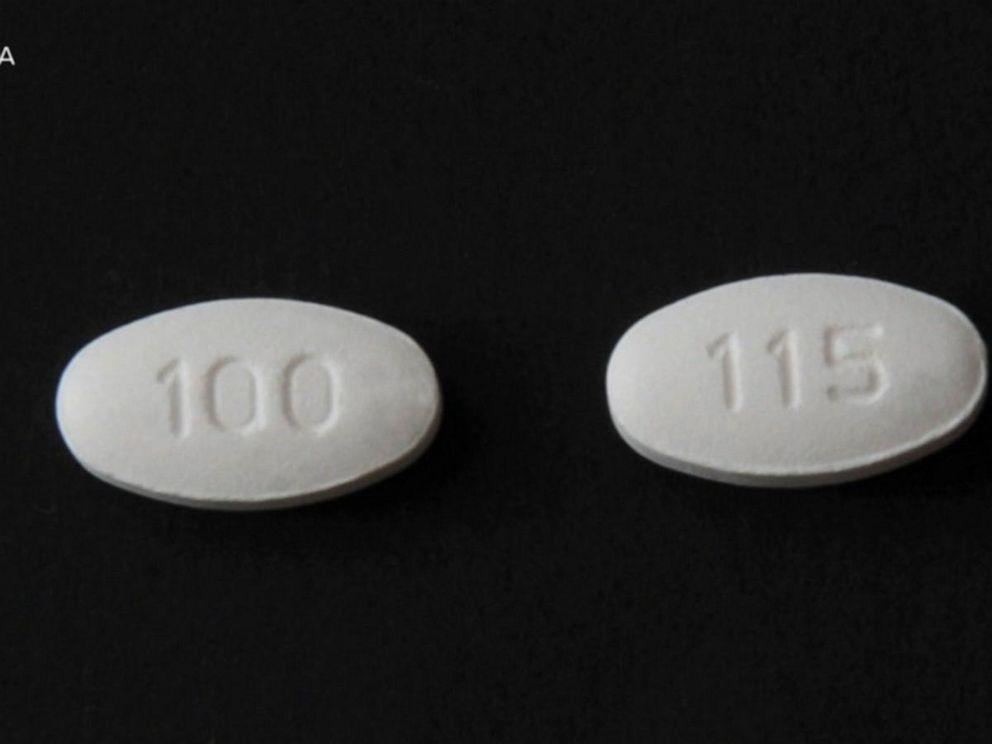


Blood Pressure Medication Recall What You Need To Know Abc News


Hydrochlorothiazide Recall After Potentially Life Threatening Mixup



Religions Free Full Text The Buddhist Medical Interface In Tibet Black Pill Traditions In Transformation Html
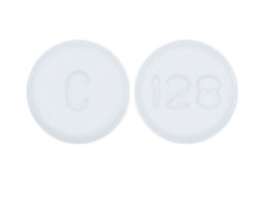


C 128 Pill Images White Round



Dea Warns On Diet Pills Kabb
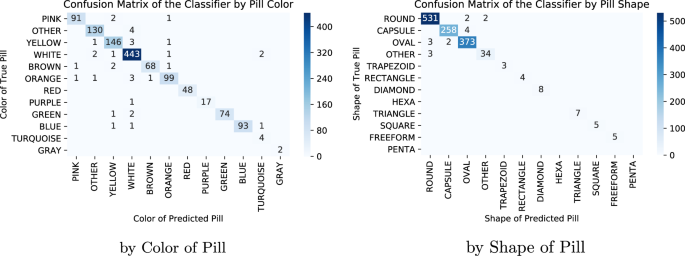


Fast And Accurate Medication Identification Npj Digital Medicine
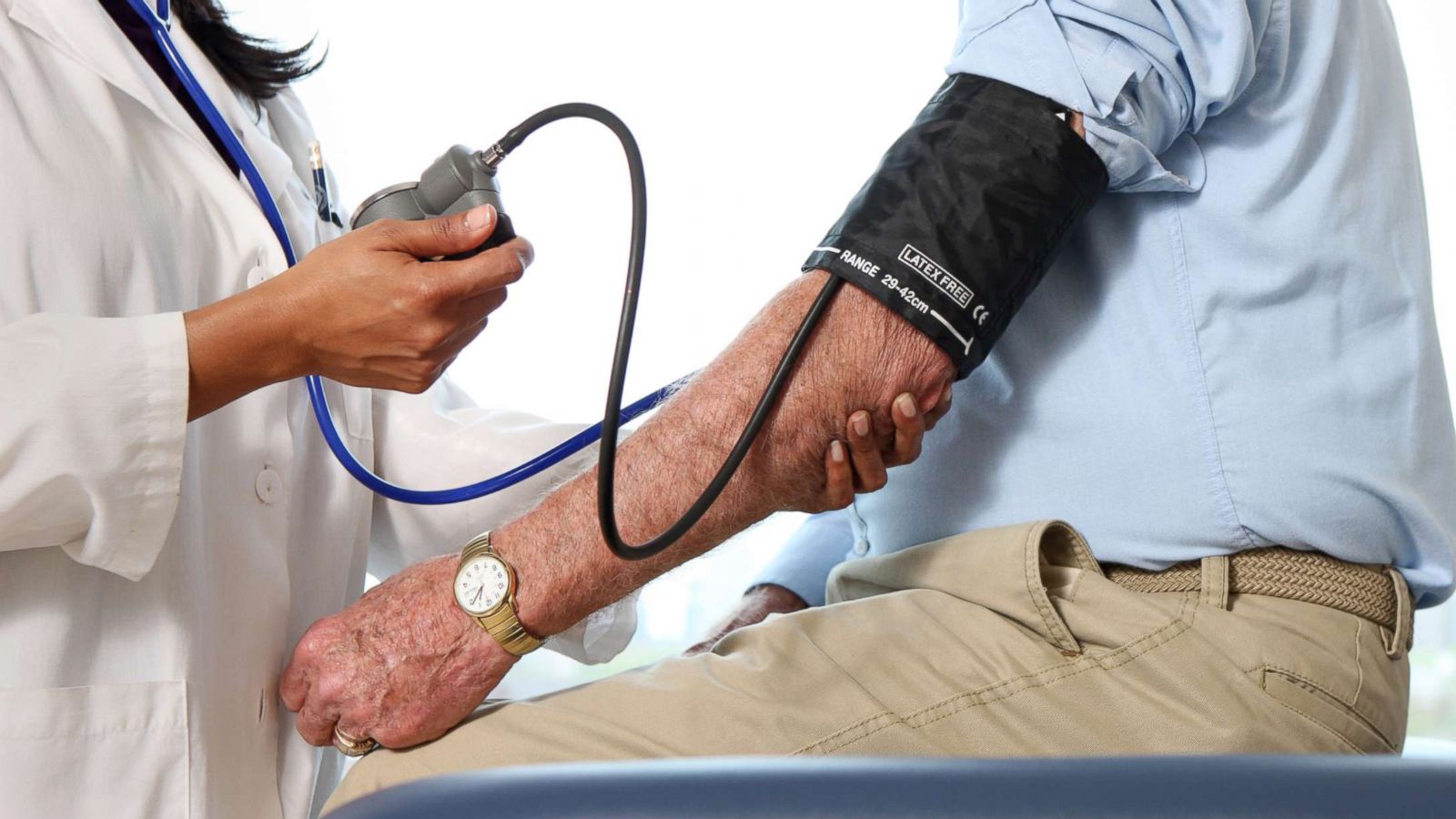


Blood Pressure Medication Recall What You Need To Know Abc News



U 128 Pill Images Orange Round


A Town For People With Chronic Fatigue Syndrome The New Yorker



Hydrochlorothiazide Recall After Potentially Life Threatening Mixup



Fda Recalls High Blood Pressure Drug Hydrochlorothiazide Fortune
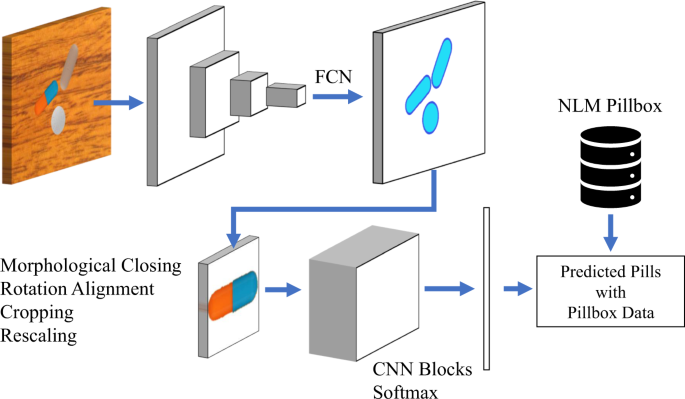


Fast And Accurate Medication Identification Npj Digital Medicine
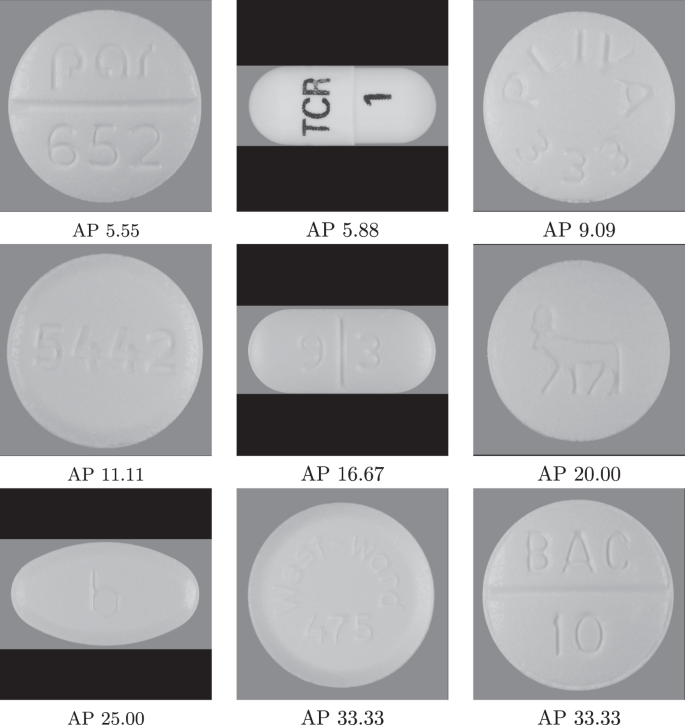


Fast And Accurate Medication Identification Npj Digital Medicine



Unichem Pharmaceuticals Usa Inc Issues A Voluntary Nationwide Recall Of Hydrochlorothiazide Tablets Due To The Potential Presence Of Foreign Tablets


Drug Reformation End Government S Power To Require Prescriptions Cato Institute
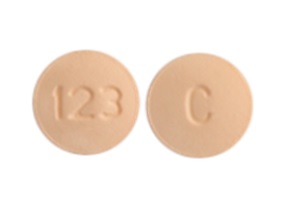


C 128 Pill Images White Round



Blood Pressure Drug Recalled Over Hydrochlorothiazide Pill Mix Up Poses Potentially Life Threatening Consequences



Recalls Spectrum News Kentucky



U 128 Pill Images Orange Round



Product Recalls Customer Service Food Lion



Religions Free Full Text The Buddhist Medical Interface In Tibet Black Pill Traditions In Transformation Html



Fda Recalls High Blood Pressure Drug Hydrochlorothiazide Fortune



Clonidine Catapres Side Effects Interactions Uses Dosage Warnings Everyday Health



Clonidine Hcl Oral Uses Side Effects Interactions Pictures Warnings Dosing Webmd
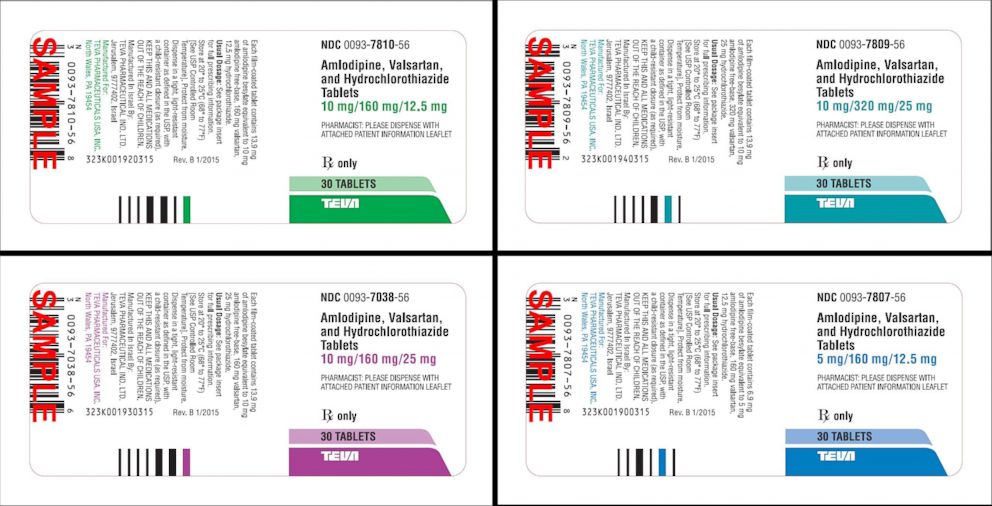


Blood Pressure Medication Recall What You Need To Know Abc News



Cryselle Norgestrel And Ethinyl Estradiol Uses Dosage Side Effects Interactions Warning


U 128 Pill Images Pill Identifier Drugs Com



Unichem Pharmaceuticals Usa Inc Issues A Voluntary Nationwide Recall Of Hydrochlorothiazide Tablets Due To The Potential Presence Of Foreign Tablets



Religions Free Full Text The Buddhist Medical Interface In Tibet Black Pill Traditions In Transformation Html


Hydrochlorothiazide Common Drug Side Effects



Hospice Medication Alert Hydrochlorothiazide 25mg Tablet Recall Hospice Pharmacy Solutions



Detecting Fake Pills With Nuclear Quadrupole Resonance Ieee Spectrum



Fentanyl Wikipedia



Abstracts From Attd 158th International Conference On Advanced Technologies Treatments For Diabetesparis France February 18 21 15 Diabetes Technology Therapeutics



U 128 Pill Images Pill Identifier Drugs Com



Diurex Ultimate Re Energizing Water Pills Walgreens


